America just keeps getting fatter and fatter — it is projected that 50% of adults will be obese and 25% will be SEVERELY obese by 2030.
For these individuals, repeating the “eat more and move less” mantra has clearly failed to work… meaning they’ll need help from proven pharmaceutical agents.
But what if there’s already a Golden Age weight loss drug on the way?
Something that simultaneously suppresses appetite better than Semaglutide, increases metabolic rate more than AOD9604, and provides the nootropic effects of a peptide like Semax?
After a month of experimentation, I’m proud to report Tesofensine as the potential all-in-one fat loss drug we’ve been searching for.
I truly love this drug and I regard it as the GOAT of fat-destroying compounds.
After one full month of usage, Monica took this image of me in Cabo San Lucas Mexico:
Read this article and you’ll see why!
MAJOR credit goes to Dr. Rudolph Eberwein of the Medical Health Institute (MHI) peptide/hormone clinic and his colleagues at the Miami-based “A New You” weight loss clinic.
They’re the ones who have brought this to my attention after unexpected success with using Tesofensine on their patients.
Table of Contents
ToggleWhat Is Tesofensine?
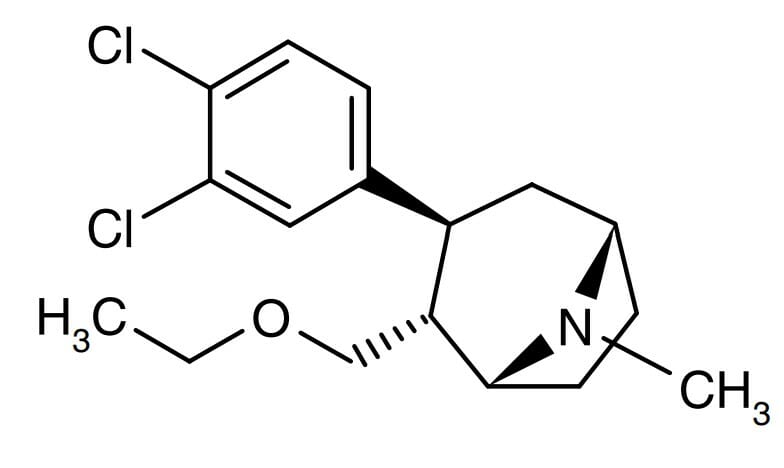
Tesofensine is a serotonin-noradrenaline-dopamine reuptake inhibitor (SNDRI) whose origins can be traced back to the development of a potential treatment for Alzheimer’s disease and Parkinson’s disease.
(Source)
The earliest mention of this drug is in 2001 under the code name “NS-2330”:
“NeuroSearch is developing NS-2330, a compound that increases the activity of dopamine, norepinephrine and acetylcholine, as a potential therapy for Alzheimer’s disease (AD) and Parkinson’s disease (PD) [260782].
It is in phase II for AD [355341], and by March 2001 was also in a phase II tolerability trial in PD patients with dyskinesia. Phase III studies are expected to begin in the third quarter of 2002 [413151].”
NeuroSearch was the Danish biotech company pursuing Tesofensine alongside Boehringer Ingelheim Corp, a laboratory based in Germany.
Tesofensine belongs to a class of drugs called “triple reuptake inhibitors” (TRIs), which were originally designed to tackle depression in a unique manner:
“…drugs acting on several monoamine systems might more effectively restore dysfunctional networks in severe and chronic disorders through mirroring the multi-system operation of the brain.
Along with greater severity and chronicity, increasingly difficult-to-treat disorder may then be refractory to monoaminergic approaches and would require the targeting of more fundamental mechanisms to restore disrupted brain networks back to normal functioning”
And given how the damage done to the systems revolving around these neurotransmitters may have something to do with Alzheimer’s and Parkinson’s, NS-2330 was pursued.
Sadly, many clinical trials (here, here), failed to have any treatment effect on advanced Alzheimer’s or Parkinson’s, and higher doses led to more frequent side effects.
But in one of the final trials for NS-2330 in the treatment of neurodegenerative diseases, weight loss was noticed as an unexpected adverse event.
And just around that time in 2006, NeuroSearch and Boehringer had a falling out:
“NeuroSearch of Copenhagen says it will go ahead with plans to study an experimental therapy (formerly in the pipeline for Alzheimer’s and Parkinson’s) after Boehringer Ingelheim dropped its rights to the compound. Boehringer halted plans for a Phase III trial of NS2330 after a mid-stage study failed to hit efficacy targets. Now NeuroSearch says it will study the compound’s effectiveness as a treatment for obesity and Type 2 diabetes in a Phase II trial slated to begin later this year”
A 2012 analysis of all these trials conclusively showed Tesofensine just couldn’t make a meaningful difference in Alzheimer’s or Parkinson’s. It was a dead end.
And this weight loss wasn’t just shedding a few pounds… it happened WITHOUT any lifestyle changes:
“…an analysis of data from 312 obese trial participants, who had either Alzheimer’s or Parkinson’s, indicated that tesofensine was, on the basis of published data, at least as effective as sibutramine, NeuroSearch said.
The subjects who received 1 mg of drug per day lost an average of 4 kg of body weight during a 14-week treatment period, which, Buus Lassen said, is shorter than most obesity studies.
‘We had no dietary restrictions, and patients were not motivated for weight loss'”
This effect was consistently observed across four different randomized, double-placebo trials within obese patients and the results were published in 2008:
“TE [tesofensine] produced a placebo-subtracted weight loss of ∼4% for >14 weeks without any diet and lifestyle therapy, which is similar to that of sibutramine, but with no effect on blood pressure. On the basis of these results, TE is now being developed for obesity management.”
Similar to the infamous story behind the accidental discovery of Viagra inducing erections as a side effect, Tesofensine also had a success emerge from what was perceived as a failure.
From that point on, NeuroSearch aggressively pursued this new path for Tesofensine… and you’ll get to see the end results if you keep reading.
But just know that outlets like ABC News were enthusiastically reporting on the results when they came out:
“‘Tesofensine produces a weight loss of approximately 10 percent more than placebo and diet in obese patients,’ said lead researcher Dr. Arne Astrup, from the Department of Human Nutrition, Faculty of Life Sciences, at the University of Copenhagen.
‘There is an enormous gap between the existing weight-loss compounds and gastric surgery… Tesofensine could close that gap. If you combine the drug with an effective diet, you could probably reach the 20 percent weight loss seen in gastric surgery.'”
Currently, Mexico-based pharmaceutical company Medix is attempting to get Tesofensine approved for the treatment of obesity, and approval is anticipated sometime in 2022.
How Does Tesofensine Work?

So how exactly does Tesofensine work across every possible facet of fat loss?
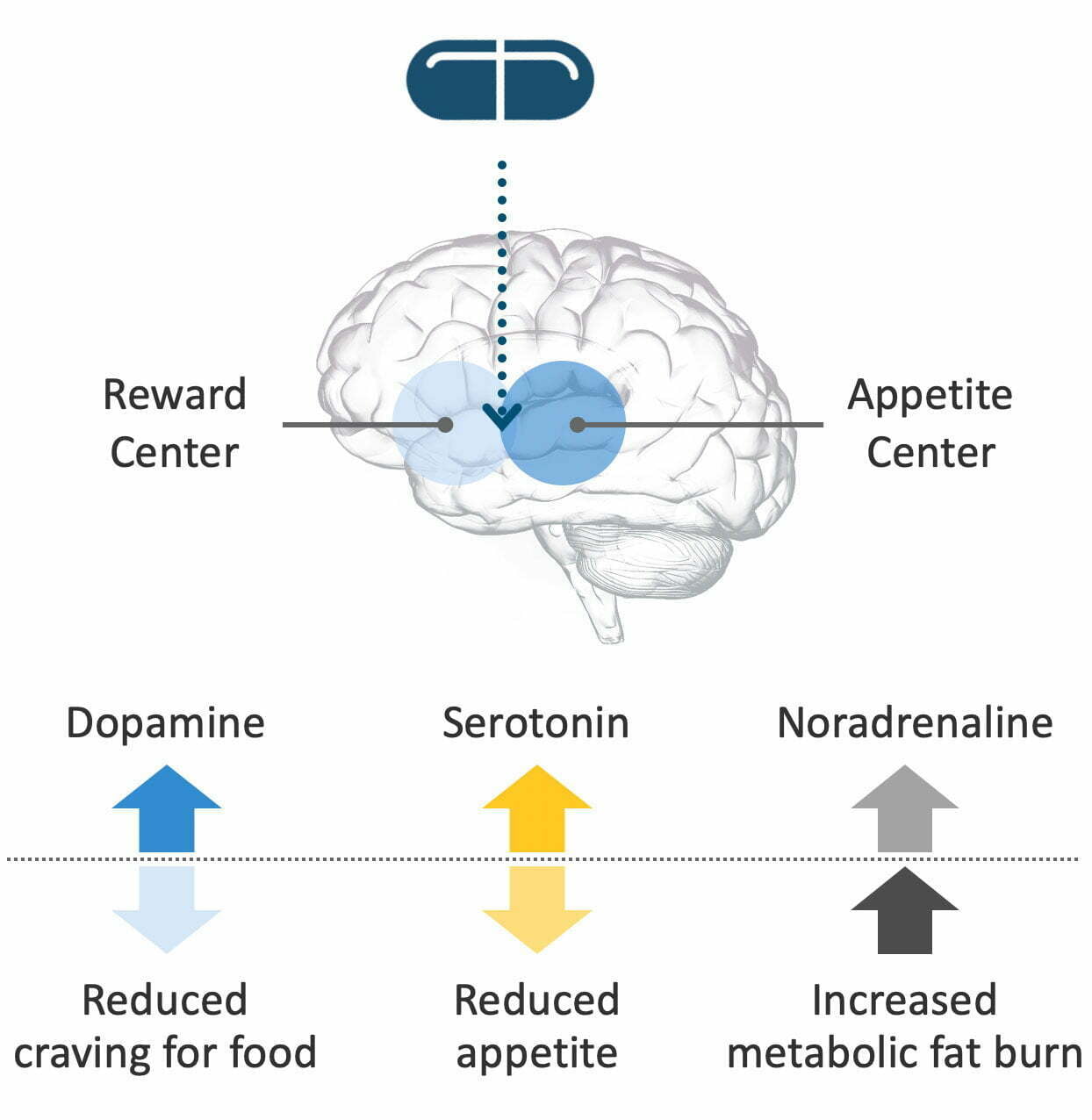
The diagram below will be useful in explaining what happens:
(Source)
Remember that Tesofensine is a reuptake inhibitor of the three neurotransmitters featured above — that means readsorption into the pre-synaptic neuron is prevented, thereby increasing the levels of the neurotransmitters in the brain and making more of them available to use.
Many mechanistic studies in rats (here, here, here, and here) have been done with respect to Tesofensine and dopamine activity, and the same for other anti-obesity drugs: At a minimum, blocking dopamine re-uptake is a fundamental part of why people feel far less ravenous after using Tesofensine.
Serotonin’s contribution towards fat loss is similar to dopamine but in a different way, so I’ll let this quote do the explaining:
“Enhancing neural serotonin release with drugs such as fenfluramine not only reduced caloric intake but also changed eating behaviour in a manner consistent with the ingestion of food. This was markedly dissimilar from the disruption to eating behaviour produced by stimulants or sedatives, or by manipulations inducing nausea, malaise or reductions in food palatability.
Consequently, it was proposed that serotonin served a critical role in the natural expression of appetite. Specifically, Blundell proposed that serotonin transmission enhanced within-meal satiation and strengthened post-meal satiety, suppressing hunger and reducing meal size”
To make the difference more clear: Increased dopamine (cravings) reduces your desire/want for specific foods, whereas increased serotonin (appetite) reduces your physical hunger NEEDS and its associated symptoms (ex. stomach growling).
As for noradrenaline, it comes in by increasing your basal metabolic rate:
“Postganglionic sympathetic neurons release noradrenaline in target organs. Via this route, lipolysis in adipose tissue is stimulated mainly via activation of b3-adrenoceptors, which are selectively expressed on adipocytes. Some of the weight-reducing effects of amphetamines may be mediated via this pathway.
b3-Adrenoceptor agonists have been considered as weight loss drugs but it has not been shown that they significantly affect body weight. Although an increased metabolic rate is observed in the first weeks of clinical treatment with b3 agonists, this effect was not sustained in the long term”
(BTW – the paper linked above is a real technical read on how all three neurotransmitters play a role in fat loss, and the weight-loss drugs that have been designed around increasing their production)
This was the technical way of explaining how a former Alzheimer’s drug such as Tesofensine could easily be repurposed into an anti-obesity solution.
Let’s see how this plays out in the real world!
Tesofensine Benefits

While it’s blatantly obvious what benefits Tesofensine provides, let’s break its fat-destroying capabilities down into specific modes of action.
After all, it’s what makes this drug so powerful for people who want to get ridiculously lean…
Weight Loss and Fat Loss
Let’s turn our attention towards the 2008 clinical trial that caused the mainstream media to make a big deal out of Tensofensine.
This was a Phase 2 randomized, double-blind, and placebo-controlled trial that involved all 203 obese patients following a calorie-restricted diet.
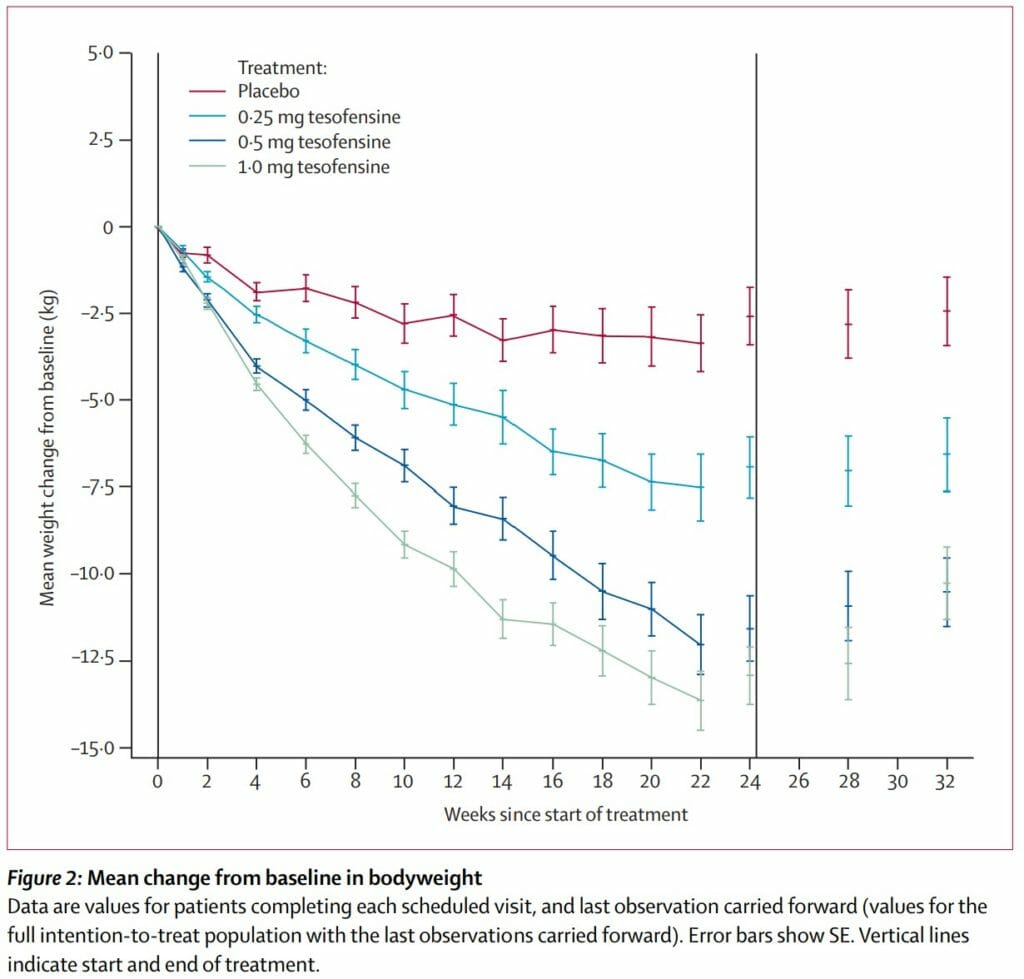
They either took a placebo, 0.25 mg, 0.5 mg, or 1 mg of tesofensine once a day for 24 weeks straight:
- The placebo group had a mean weight loss of 2%
- The 0.25 mg Tesofensine group had a mean weight loss of 4.5%
- The 0.5 mg Tesofensine group had a mean weight loss of 9.2%
- The 1.0 mg Tesofensine group had a mean weight loss of 10.6%
And if you think that’s a big deal, just wait until you see what the lead author presented at a conference that same year:
“The Baecke physical activity questionnaire showed that TE [tesofensine] increased the time patients spent walking and reduced the time spent watching TV compared to placebo.
The 2 highest doses of TE significantly affected appetite measures compared with placebo as assessed by VAS, with a 3 to 4 fold reduction in hunger (P = 0.001), and a reduction in expected next meal size (P < 0.002). Desires for sweet, fatty or salty foods were reduced in the 2 highest dose groups.
Quality of Life (assessed by IWQoL-Lite) found consistent beneficial effects of TE compared to placebo. Significant improvements were seen in the “Overall score” and domains of “Physical Function” and “Self-Esteem” (P<0.05), with modest improvements in “Sexual Life”, “Public Distress” and “Work”.”
Twice the amount of induced weight loss compared to existing drugs on the market or in the middle of testing… it was truly a sight to behold:
“Current anti-obesity medications on the market produce a weight loss that is 3-5 kg greater than placebo over 6 months, and the major pharmaceutical companies have for several years been struggling to develop drugs that produce a 6-8 kg weight loss. Therefore, I was thrilled to see that the tesofensine trial actually produced a weight loss of approx. 10 kg more than placebo, without major safety concerns.”
Just to put this in context: “For the 0.5 mg and 1.0 mg doses, this represented a weight loss around twice that attained using sibutramine (Reductil®/Meridia®) or rimonabant (Accomplia®), the currently-approved therapies in Europe – and in half the treatment time” (Source).
And it was the RIGHT kind of weight loss:
“…dose-related reductions in body weight, body fat and waist circumference, as well as improvements in other obesity-related endocrine factors, were observed.”
“The initial positive findings suggest that tesofensine may be a well-tolerated long-term treatment for obesity, with minimal cardiovascular effects; this view appears to be shared by the FDA, which recently endorsed the phase III trial program for the agent.”
If 24 weeks seems like too big of a time period for you, let’s look at what happens over a period of 14 days:
“In TIPO-2, 32 overweight volunteers (BMI of 28–35) were randomised to receive tesofensine (n = 16) or placebo, for 14 days; there were no diet or exercise interventions and patient were urged not to change their lifestyle.
The tesofensine recipients achieved a mean weight loss of 2.2kg, compared with 0.4kg in the placebo recipients; the difference was significant. The maximum weight loss was 4.7kg”
Even without a calorie-restricted diet in place, or an exercise program, Tesofensine still outperformed a regular lifestyle with respect to weight loss!
And guess what… the weight loss is fully sustained:
“In the TIPO-4 trial, a 48-week open-label extension to the TIPO-1 trial, preliminary results suggest that weight loss with tesofensine is sustained. After an initial eight-week washout period, patients continuing with 0.5mg tesofensine achieved a total mean weight loss of 13–14kg at 24 weeks.
Additionally, previous placebo recipients who switched to tesofensine 0.5mg lost approximately 9kg over the same period.”
It gets better — in 2018, Denmark-based pharmaceutical company Saniona reported excellent Phase 3 trial results with Tesofensine alone:
“The 24-week double-blinded, randomized, placebo-controlled trial investigated the efficacy and safety of once-daily 0.25 and 0.50 mg oral tesofensine compared to placebo in 372 obese patients. The study’s primary endpoint was the average percentage and absolute change in body weight compared to placebo. Secondary endpoints included the percentage of patients achieving weight loss of at least five per cent and ten percent of baseline body weight.”
At week 24, both treatment groups obtained highly statistically and clinically significant reductions on all major efficacy endpoints compared to placebo including average percentage and absolute change in body weight, reduction in BMI and the proportion of patients achieving weight loss of at least five 5% and 10% of baseline body weight [More than half of patients lost more than 10% in weight].
Statistically and clinically significant reductions in obesity-related risk factors were also observed in tesofensine-treated participants compared with those receiving placebo including, waist circumference, hip circumference, body fat, visceral fat, very-low-density lipoprotein (“bad”) cholesterol, triglycerides and insulin.”
Let’s see how else Tesofensine can help make the fat loss journey even easier…
Increases BDNF Production
One of the first things I noticed with Tesofensine was the increased brain-derived neurotrophic factor (BDNF) production, especially while I was fasting for extended periods of time.
My energy was higher, my mood was uplifted, and my focus was enhanced — overall the quality of my life was so much better (and I slept better too).
But this isn’t a surprise to anybody who’s read The Metabolic Blowtorch Diet and KNOWS the cognitive-enhancing powers of BDNF!
As it turns out, Tesofensine upregulates the expression of BDNF in the brain:
“We find that chronic [14 days], but not sub-chronic [5 days] treatment with Tesofensine increases BDNF mRNA in the CA3 region of the hippocampus (35%), and Arc mRNA in the CA1 of the hippocampus (65%). Furthermore, the number of Ki-67- and neuroD-positive cells increased after chronic, but not sub-chronic treatment.
This study shows that Tesofensine enhances hippocampal gene expression and new cell formation indicative for an antidepressant potential of this novel drug substance”
Seeing how BDNF itself has an anti-depressant effect, this finding isn’t all that surprising.
Suppresses Appetite
There’s no point in having greater weight loss if you feel ravenous and eat enough to put back all the weight on.
In 2012, rats with diet-induced obesity were taking 2.0 mg/kg of Tesofensine, 2.0 mg/kg of Tesofensine plus a 28-day period of no treatment, a delivery vehicle, or a delivery vehicle plus a restricted diet matching the very first group.
And as we saw in the previous studies, it should be no surprise that the rats ate significantly less:
“Caloric intake and weight gain decreased significantly more in the tesofensine-treated rats compared to vehicle-treated rats, which confirms previous findings. After treatment discontinuation, caloric intake and body weight gain gradually increased again.
Tesofensine-treated rats showed significantly lower D2/3R availability in nucleus accumbens and dorsal striatum than both vehicle-treated rats and vehicle-treated rats on restricted isocaloric diet… Thus, chronic tesofensine treatment leads to decreased food intake and weight gain.”
And in case you’re wondering what the whole ‘D2/3R’ thing means, the abstract sums it up for you:
“Obesity is associated with lower striatal dopamine D2 receptor availability, which may be related to disturbed regulation of food intake. This study assesses the effects of chronic tesofensine treatment on food intake and body weight in association with changes in striatal dopamine D2/D3 receptor (D2/3R) availability of diet-induced obese (DIO) rats”
It’s unfortunate to see the rats re-gaining weight and eating more after stopping the use of Tesofensine, but it’s what you would expect for a weight loss drug.
Fortunately, this same effect was replicated in humans within the same year.
This was a two-part Phase 2 trial where the first part involved a similar setup to the pivotal 2008 clinical trial mentioned earlier, and the second part involved taking 0.5 or 1.0 mg of Tesofensine by subjects who wished to continue participating in the study.
While satiety increased in the early stages, it was interesting to see it gradually wane over time:
“In Part 1 TE [tesofensine] induced a dose-dependent increase in CSS [composite satiety score] at week 12 that correlated with weight loss during the 24 weeks (r = 0.36, P < 0.0001). However, CSS diminished over time as weight loss progressed (e.g., for 1.0 mg; 52 ± 17 mm; 64 ± 13 mm; 55 ± 13 mm at baseline, week 12 and week 24, respectively).
After drug withdrawal CSS returned to baseline values (50 ± 17 mm, in the whole sample.), despite the participants’ reduced-weight state (-7.2 ± 6.7 kg, P < 0.0001). The reintroduction of TE in Part 2 increased CSS again (56 ± 17 mm at week 60), regardless of initial treatment/weight loss”
Either Tesofensine has an appetite-suppressing effect that diminishes due to tolerance, or the new “state” of the individual counteracts the actions of Tesofensine.
It’s hard to say for sure, but it’s a good sign that Tesofensine shouldn’t be used any longer than necessary.
Enhances Metabolic Rate
One of the ways in which Tesofensine allows people to lose fat faster is through increased thermogenesis (i.e. energy expenditure that’s higher than your normal metabolic rate).
This is exactly what researchers found in a small 2010 human trial involving 32 men who were obese, overweight, or healthy.
Without changing their normal exercise routine or diet, they took 2 mg of Tesofensine every day for a week followed by placebo or 1.0 mg of Tesofensine in the second weight.
After keeping track of body composition and energy expenditure, here’s what they found:
“Despite efforts to keep body weight and composition constant, TE [tesofensine] induced a 1.8 kg weight loss above PL [placebo] after 2 weeks’ treatment (P<0.0001). TE also induced higher ratings of satiety and fullness and concomitantly lower prospective food intake than placebo.
No significant effect of TE on total 24-h EE could be demonstrated compared with PL, but higher energy expenditure was observed during the night period (4.6%; P<0.05) when adjusted for changes in body composition. Furthermore, TE increased 24-h fat oxidation as compared with PL (18 g; P<0.001).
TE has a pronounced effect on appetite sensations and a slight effect on energy expenditure at night-both effects can contribute to the strong weight-reducing effect of TE.”
It certainly explains the rapid fat loss observed in 2008 that made headlines…
Improves Critical Biomarkers
Finally, what’s the point of losing weight if your health also takes a beating?
Tesofensine isn’t your average risky weight-loss drug that kills you in the process.
Even in rat studies, we can see that insulin production and lipid levels are better handled with the use of Tesofensine:
“…the hypophagic effect of tesofensine was longer lasting than [weight loss drugs] sibutramine and rimonabant. In contrast to tesofensine, the body weight of pair-fed rats returned to baseline at the end of the study, which may indicate that tesofensine stimulated energy expenditure.
The differential efficacy on weight reduction was also reflected in lowered body fat depots, as tesofensine and sibutramine most efficiently reduced abdominal and subcutaneous fat mass which was paralleled by reduced plasma lipid levels.
In an oral glucose tolerance test, only tesofensine significantly suppressed the plasma insulin response below the level that could be obtained by paired feeding, indicating that tesofensine further improved glycemic control.
In conclusion, the robust weight loss with long-term tesofensine treatment is likely due to a combined synergistic effect of appetite suppression and increased energy expenditure.”
Even in the game-changing 2008 clinical trial I keep referring back to, Tesofensine was “found to have beneficial effects on plasma insulin and HbA1c concentrations” (Source).
Huge implications for Type 2 diabetics!
Tesofensine Dosage for Weight Loss

To hype up Tesofensine even more, it comes in the form of a convenient capsule/tablet and nothing else is needed.
Simply take one 0.5 mg tablet first thing in the morning while fasted every day until your fat loss cycle is complete, and then stop taking it.
An obese individual may need to go up to two tablets (1 mg total) per today, although the lead author of the pivotal 2008 clinical trial, Dr. Arne Astrup, insists 0.5 mg is good enough:
“Twelve percent of the 0.5-mg group did not complete the study, as compared with 29% of the 1.0-mg group and 25% of the placebo group. Of the patients who dropped out of the study’s 1.0-mg arm, 20.4% did so because of adverse effects, compared with a combined average of 8% in the other groups.
Dr. Astrup suggested that the optimal dose for tesofensine may be 0.5 mg. ‘There’s no reason to pursue [a potential dose of 1.0 mg],” he noted, “because it doesn’t produce much more weight loss, but [it] increases problems.'”
Keep in mind that this recommended dosing for Tesofensine exists despite its abnormally long half-life of 234 hours (~9-10 days).
It most likely explains why people can still feel satiated a week after Tesofensine use is stopped but then gradually feel hungry again.
Tesofensine Side Effects and Safety
Tesofensine has some side effects you should know about before you start using it recklessly.
There have been several critiques written about the 2008 clinical trial, one of which involves the increase of blood pressure among Tesofensine users:
“Decreases in blood pressure of 3–5 mm Hg systolic and 2–3 mm Hg diastolic are seen with weight losses of 4–5 kg achieved through weight loss and dietary modification.
In Astrup and colleagues’ trial, blood pressure increased by 1 mm Hg (systolic) and 2–3 mm Hg (diastolic) among participants taking tesofensine 0·25 or 0·5 mg compared with controls. Thus, tesofensine seems to increase both systolic and diastolic blood pressure by 4–6 mm Hg when compared with a similar weight loss achieved by lifestyle modification alone. An increase in blood pressure due to tesofensine is not surprising, given the mechanism of action of the drug.”
It seems as if there is a dose-dependent effect in which higher doses of Tesofensine increase the frequency and severity of this side effect:
“Heart rate was significantly elevated in all tesofensine groups (5 to 8 beats per min). The 0.25 dose and the 0.5 mg dose did not show any changes in blood pressure as compared to placebo; however, the highest dose of tesofensine of 1.0 mg daily was associated with a significant increase in systolic and diastolic blood pressure (mean increase 6.8/5.8 mmHg).
Insomnia, dry mouth, constipation, diarrhea, and dizziness were also more common. The highest dose of tesofensine showed the highest frequency of mood change. These mild increases in heart rate or blood pressure may end up having clinical significance, as was recently seen in sibutramine.”
(NOTE: NeuroSearch insists Tesofensine is safe for the heart using this small study where no increases in blood pressure were observed while subjects were in situations that stressed the cardiovascular system)
A deeper look suggests “tesofensine 1.0 mg increased anger and hostility and both 0.5 mg and 1.0 mg of tesofensine increased the risk of confusion” (Source).
Some scientists have speculated that Tesofensine has high abuse potential due to its ability to increase dopamine levels in the brain and exert stimulant effects (i.e. similar to how cocaine’s effects are mediated).
However, this notion has been put to rest:
“A concern with drugs that block DA [dopamine] transporters is their potential for abuse. This has been addressed in a study by Schoedel et al. They analyzed the abuse potential of the triple reuptake inhibitor tesofensine, compared with placebo, d-amphetamine, bupropion and atomoxetine in recreational stimulant users.
The effects of tesofensine were not significantly different from those of placebo. The results showed that, certainly for the triple uptake inhibitor tesofensine, blockade of DA uptake appears unlikely to be associated with recreational abuse.”
The possibility of psychiatric reactions has also been raised, but the lead author of the 2008 clinical trial has assured the medical community that nothing of the sort was observed:
“We saw no haemolysis or serious psychiatric adverse reactions (agitation, panic attacks, mood disorders) even though we included patients with a previous history of anxiety or depression. All drugs have adverse effects, and the challenge is to develop medications with an acceptable efficacy–safety profile.
The phase III programme for tesofensine, with prospective psychiatric and cardiovascular endpoints, which could be followed-up by a meta-analysis of the events, will provide better evidence of its long-term effect on morbidity and of its safety. But to request trials with hard endpoints such as mortality before launch would prevent any weight-loss compounds reaching the market”
Finally, an investigation in 2011 at two of the trial sites used to generate data for the 2008 clinical trial reveal possible under-reporting of adverse events:
“Three major points were raised by the inspection.
First, Danish regulations require that informed consent is undertaken in its entirety by a medical practitioner, but in one site this duty had been delegated to non-medical personnel.
Second, the integrity of blinding was questioned in an earlier inspection in 2007, and rebutted by the investigators.
Third, the recording and assessment of adverse events by the contract research organization was incomplete, because their staff did not consider as noteworthy the recurrence of events that trial participants had experienced on occasions prior to the study.
In particular, headache, migraine, stress, and depression were not reliably recorded in people who had experienced these conditions before enrolling in the trial. Therefore, the report concluded that “the overall side-effect profile that is…published in The Lancet is not in accordance with the actual course of the trial””
Interpret that how you will, but Tesofensine wouldn’t still be around today if those discoveries were enough to banish the drug into complete non-existence.
In summation, here are the side effects to watch out for when using Tesofensine:
- Elevated heart rate
- Increased blood pressure
- Nausea
- Insomnia / sleep disturbances
- Diarrhea
- Constipation
- Dry mouth
- Mood swings involving anger
Do your due diligence, be responsible, start at the lowest dose and test it before going higher, and always speak to a physician if you are unsure about the use of Tesofensine.
Where To Buy Tesofensine Online

UPDATE, AUGUST 12 2022: Limitless Life Nootropics officially has Tesofensine available for pre-order.
Priced at $220 for a bottle of thirty 0.5 mg tablets, they are by far the best (and only) place to purchase Tesofensine online.
Don’t forget to use code JAY15 for 15% off your order!
Limitless Life Nootropics currently does not hold this product in stock, otherwise I would direct you there.
Your best bet for purchasing Tesofensine is to visit Peptide Sciences and order a few bottles.
They’re my second choice behind Limitless and are a favorite among biohacking enthusiasts worldwide for numerous reasons — long-standing reputation, excellent customer support, affordable prices, and worldwide shipping.
One bottle of thirty 0.5 mg tablets will be enough to last you a month… at $250 per bottle, this isn’t something you should use unless you (a) have the budget and (b) are already lean for best results.
It’s also a non-prescription drug at the moment, but who knows how long this freedom will last!
Thoughts on Tesomet
I’ll try to keep this as brief as possible to avoid unnecessary writing, but Tesomet is a dual combination of Tesofensine and the drug Metropolol (used to treat high blood pressure).
Back in 2013, researchers discovered that using Metropolol could help counteract Tesofensine’s side effect of increasing blood pressure without interfering with appetite suppression:
“Acute administration of tesofensine caused a dose-dependent hypophagic effect as well as increased heart rate and blood pressure. Interestingly, combined treatment with metoprolol (b1 adrenoceptor blocker, 10-20 mg/kg, p.o.) fully prevented the cardiovascular sympathetic effects of tesofensine while leaving the robust inhibitory efficacy on food intake unaffected.”
2017 was when this beneficial effect was also observed in overweight/obese Type 2 diabetics (albeit in the form of a conference paper).
Tesomet is being pursued by Danish pharmaceutical company Saniona, who is currently studying the combination for two rare diseases:
- Hypothalamic obesity (Phase 2b trial data expected in second half of 2023): A damaged hypothalamus fails to function normally, dysregulating hormones associated with hunger and leading to symptoms such as uncontrollable cravings, compromised metabolic rate, and unusually fast/excessive weight gain
- Prader-Willi Syndrome (Phase 2b trial data expected in first half of 2023): A genetic disorder where the individual is in a constant state of hunger and never feels satiated, alongside food hoarding, leading to excessive weight gain (among other features such as cognitive impairment and poor physical development)
The FDA granted orphan drug status to Tesomet for hypothalamic obesity in July 2021, and did the same for Prader-Willi syndrome in March 2021.
For the former, a randomized controlled trial published in 2021 found Tesomet did not affect heart rate, was well-tolerated, and an average weight loss of 6.3% was achieved over 24 weeks in 21 patients with the condition (not to mention seeing a lower waist circumference and more fat loss than lean muscle loss)
As for Prader-Willi Syndrome, the Phase 2a trial results look promising:
“This was an exploratory, randomized, double-blind, placebo-controlled Phase 2a trial in 18 patients with PWS, which was divided into two parts; the first was performed in nine adult patients with PWS and the second in nine growing adolescent patients. The primary endpoint was to examine the change in body weight with Tesomet compared to placebo. Secondary objectives included eating behaviour and food craving (hyperphagia), body composition, lipids and other metabolic parameters.
The first part was successfully concluded and first reported in 2018. The results showed that Tesomet 0.5 mg/day for three months provided clinically meaningful weight loss and a significant reduction in hyperphagia in adult patients. The study results also suggested that the optimal dose of Tesomet in patients with PWS may be lower than in other indications such as hypothalamic obesity.”
That’s all you need to know — relevant for people suffering from these rare conditions, but not for the fully optimized biohacker.
Additional Reading Resources For Tesofensine

The bad news is this: While Tesofensine is in the midst of collecting more trial data for full FDA approval, we don’t have much more to rely on with regards to hard data.
So while we’re waiting on our hands, here are two resources if you want to learn even more about this groundbreaking fat loss drug.
This 5-minute summary from Genemedics covers 58 studies involving Tesofensine directly or indirectly, covering benefits not featured in this article that include deeper sleep, reduced alcohol addiction, treatment of sexual dysfunction, and more!
This website keeps track of the ongoing Tesomet/Tesofensine clinical trials, and even shows you a history of how many companies the drug has been involved with since 2003.
As always…
Raise Your Vibration To Optimize Your Love Creation!
PS – If you want to be in my private circle and get access to Golden Age agents like Tesofensine several months before I ever speak about them publicly, join The Fully Optimized Health Private Membership Group.
It’s your greatest opportunity to fully optimize your health, gain total access to myself and network with high-level men and women looking to 10X their life.




